- Clinical Study
- Clinical Outcomes after Early and Delayed Radioiodine Remnant Ablation in Patients with Low-Risk Papillary Thyroid Carcinoma: Propensity Score Matching Analysis
-
Jonghwa Ahn, Meihua Jin, Eyun Song, Min Ji Jeon, Tae Yong Kim, Jin-Sook Ryu, Won Bae Kim, Young Kee Shong, Ji Min Han, Won Gu Kim
-
Endocrinol Metab. 2020;35(4):830-837. Published online November 18, 2020
-
DOI: https://doi.org/10.3803/EnM.2020.747
-
-
4,241
View
-
132
Download
-
3
Web of Science
-
6
Crossref
-
 Abstract Abstract
 PDF PDF Supplementary Material Supplementary Material PubReader PubReader  ePub ePub
- Background
The clinical outcomes of delayed radioiodine remnant ablation (RRA) therapy in patients with low-risk papillary thyroid carcinoma (PTC) are unclear. We aimed to evaluate the clinical impact of the interval between total thyroidectomy (TT) and RRA therapy in patients with low-risk PTC.
Methods
We included 526 patients who underwent TT and RRA for low-risk PTC with a primary tumor size of >1 cm between 2000 and 2012. Patients were divided into the early (<90 days) and the delayed (≥90 days) RRA groups based on the interval between TT and RRA. The results of diagnostic whole-body scan (DxWBS), ongoing risk stratification (ORS; response to therapy), and disease-free survival (DFS) were evaluated before and after propensity score matching (PSM).
Results
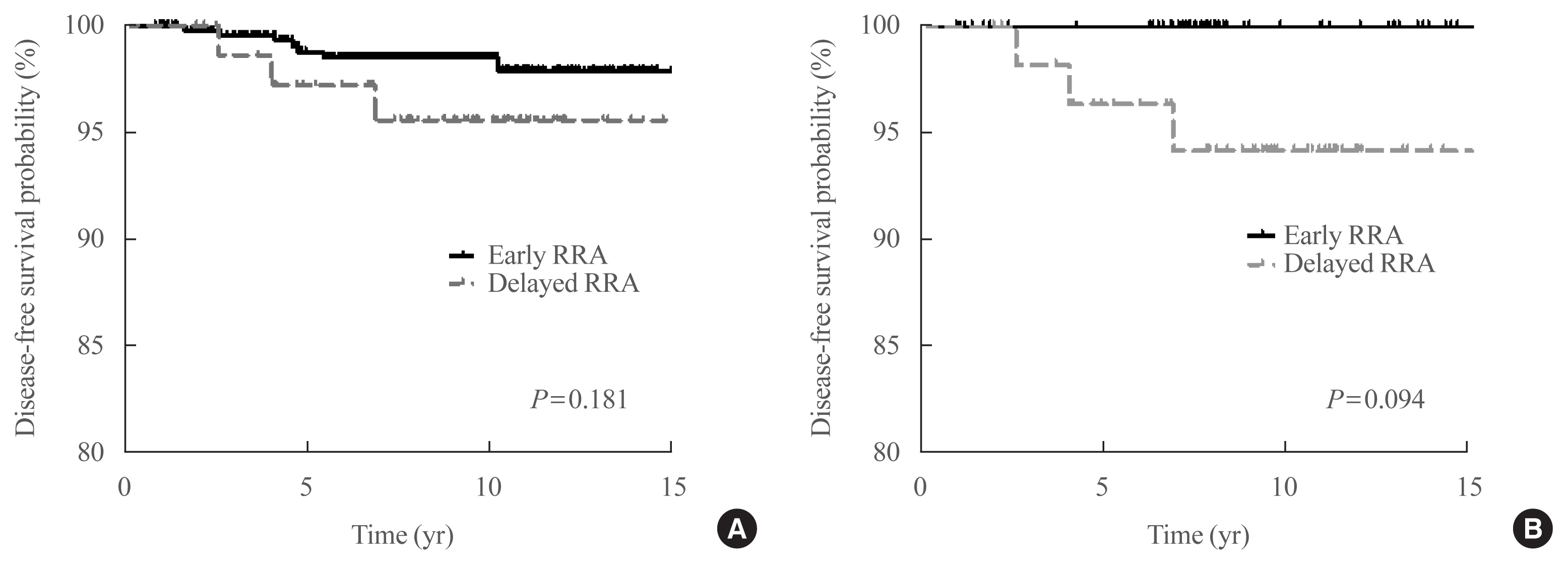
Among the 526 patients, 75 (14.3%) patients underwent delayed RRA; they had more cervical lymph node metastasis and received a higher RRA dose than those who underwent early RRA. The median follow-up period was 9.1 years after initial therapy, and the structural recurrence rate was 1.9%. In DxWBS, 60 patients had focal iodine uptake limited in operative bed, with no significant difference between groups. According to ORS, 78%, 20%, 1%, and 1% patients were classified into excellent, indeterminate, biochemical incomplete, and structural incomplete response groups, respectively. There was no significant difference in ORS or DFS between groups before and after PSM.
Conclusion
The timing of the first RRA had no clinical impact in patients with low-risk PTC. Thus, the clinical decision for RRA can be determined >3 months after TT considering other prognostic factors.
-
Citations
Citations to this article as recorded by  - Dynamic risk assessment in patients with differentiated thyroid cancer
Erika Abelleira, Fernando Jerkovich
Reviews in Endocrine and Metabolic Disorders.2024; 25(1): 79. CrossRef - Ablation Rates and Long-Term Outcome Following Low-Dose Radioiodine for Differentiated Thyroid Cancer in the West of Scotland: A Retrospective Analysis
Kathryn Graham, Fay Tough, Helena Belikova, Irene Wotherspoon, David Colville, Nicholas Reed
Endocrine Practice.2024; 30(4): 327. CrossRef - Radioiodine ablation after thyroidectomy could be safely abandoned or postponed in selected stage I papillary thyroid carcinoma patients of low-risk group: an observational prospective study
S.M. Cherenko, A.Yu. Glagolieva, D.E. Makhmudov
INTERNATIONAL JOURNAL OF ENDOCRINOLOGY (Ukraine).2024; 20(1): 7. CrossRef - Patient Preparation and Radiation Protection Guidance for Adult Patients Undergoing Radioiodine Treatment for Thyroid Cancer in the UK
J. Wadsley, N. Armstrong, V. Bassett-Smith, M. Beasley, R. Chandler, L. Cluny, A.J. Craig, K. Farnell, K. Garcez, N. Garnham, K. Graham, A. Hallam, S. Hill, H. Hobrough, F. McKiddie, M.W.J. Strachan
Clinical Oncology.2023; 35(1): 42. CrossRef - Delay of initial radioactive iodine therapy beyond 3 months has no effect on clinical responses and overall survival in patients with thyroid carcinoma: A cohort study and a meta‐analysis
Fang Cheng, Juan Xiao, Fengyan Huang, Chunchun Shao, Shouluan Ding, Canhua Yun, Hongying Jia
Cancer Medicine.2022; 11(12): 2386. CrossRef - Delayed (>3 Months) Postoperative Radioactive Iodine Ablation Does Not Impact Clinical Response or Survival in Differentiated Thyroid Cancers
Tatiana Fedorova, Lilah F. Morris-Wiseman
Clinical Thyroidology.2022; 34(10): 456. CrossRef
- Clinical Study
- Clinical Outcomes of N1b Papillary Thyroid Cancer Patients Treated with Two Different Doses of Radioiodine Ablation Therapy
-
Meihua Jin, Jonghwa Ahn, Yu-Mi Lee, Tae-Yon Sung, Won Gu Kim, Tae Yong Kim, Jin-Sook Ryu, Won Bae Kim, Young Kee Shong, Min Ji Jeon
-
Endocrinol Metab. 2020;35(3):602-609. Published online September 22, 2020
-
DOI: https://doi.org/10.3803/EnM.2020.741
-
-
5,403
View
-
121
Download
-
1
Web of Science
-
 Abstract Abstract
 PDF PDF PubReader PubReader  ePub ePub
- Background
The optimal dose of radioactive iodine (RAI) therapy for N1b papillary thyroid carcinoma (PTC) is controversial. We evaluated the clinical outcome of N1b PTC patients treated with either 100 or 150 mCi of RAI.
Methods
We retrospectively analyzed N1b PTC patients who underwent total thyroidectomy and postoperative RAI therapy at a tertiary referral center between 2012 and 2017. As the baseline characteristics differed between treatment groups, we performed exact matching for various pathological factors according to RAI dose. We evaluated the response to therapy and recurrence-free survival (RFS) in the matched patients. Structural recurrent/persistent disease was defined as new structural disease detected after initial therapy, which was confirmed by cytology or pathology.
Results
Of the total 436 patients, 37 (8.5%) received 100 mCi of RAI and 399 (91.5%) received 150 mCi of RAI. After an exact 1:3 matching, 34 patients in the 100 mCi group and 100 patients in the 150 mCi group remained. There was no significant difference in response to therapy between the groups in the matched population (P=0.63). An excellent response was achieved in 70.6% (n=24) of patients in the 100 mCi group and 76.0% (n=76) in the 150 mCi group. Two (5.9%) patients in the 100 mCi group and four (4.0%) in the 150 mCi group had recurrence and there was no significant difference in RFS between the groups in the matched population (P=0.351).
Conclusion
There were no differences in response to therapy and RFS in N1b PTC patients according to RAI dose.
- Endocrine Research
- Comparison of Thyroglobulin Measurements Using Three Different Immunoassay Kits: A BRAMHS Tg-Plus RIA Kit, a BRAMHS hTg Sensitive Kryptor Kit, and a Beckman Coulter ACCESS Immunoassay Kit
-
Mijin Kim, Min Ji Jeon, Won Gu Kim, Jong Jin Lee, Jin-Sook Ryu, Eun-Jung Cho, Dae-Hyun Ko, Woochang Lee, Sail Chun, Won-Ki Min, Tae Yong Kim, Young Kee Shong, Won Bae Kim
-
Endocrinol Metab. 2016;31(3):462-468. Published online August 2, 2016
-
DOI: https://doi.org/10.3803/EnM.2016.31.3.462
-
-
5,051
View
-
49
Download
-
8
Web of Science
-
7
Crossref
-
 Abstract Abstract
 PDF PDF PubReader PubReader
- Background
Second-generation thyroglobulin immunometric assays (Tg-IMAs) have been developed with improved sensitivity. Our aim was to compare the diagnostic value of Tg-IMA measurements using a Kryptor (BRAHMS AG) kit (Tg-K) and an ACCESS (Beckman Coulter) kit (Tg-A) with that of the first-generation Tg measurement using a Tg-plus (BRAHMS AG) kit (Tg+). MethodsWe enrolled 82 differentiated thyroid cancer patients who underwent total thyroidectomy with radioactive iodine remnant ablation and who underwent diagnostic whole body scan using recombinant human thyroid stimulating hormone (rhTSH). The Tg+, Tg-K, and Tg-A were measured before rhTSH administration during levothyroxine treatment (suppressed Tg) from the same sample. Serum Tg+ was measured after rhTSH stimulation (stimulated Tg). ResultsSuppressed Tg+ was more significantly correlated with suppressed Tg-K (R2=0.919, P<0.001) than with suppressed Tg-A (R2=0.536, P<0.001). The optimal cut-off values of suppressed Tg+, Tg-K, and Tg-A for predicting stimulated Tg+ of 1 ng/mL were 0.3, 0.2, and 0.2 ng/mL, respectively. The sensitivity, specificity, and accuracy of suppressed Tg+ were 67%, 100%, and 90%, respectively; those of suppressed Tg-K were 83%, 90%, and 88%; those of suppressed Tg-A were 96%, 82%, and 87%, respectively. The positive predictive and negative predictive values of Tg+ were 100% and 87%, respectively; those of Tg-K were 79% and 92%; and those of Tg-A were 73% and 98%. ConclusionWe could not clearly demonstrate which kit had better diagnostic performance after comparison of first-generation Tg measurements with Tg-IMA measurements. Also, there were kit-to-kit variations between Tg-IMA kits. Suppressed Tg measured by Tg-IMA was insufficient to completely substitute for a stimulated Tg measurement.
-
Citations
Citations to this article as recorded by  - Comparison of the diagnostic performances of US-guided fine needle aspiration cytology and thyroglobulin measurement for lymph node metastases in patients with differentiated thyroid carcinoma: a meta-analysis
Rong-Bin Liu, Da-Lei Zhou, Bo-Heng Xu, Xin-Hua Yang, Qing Liu, Xiao Zhang, Tao Tang, Zu-Lu Ye, Yue Li
European Radiology.2021; 31(5): 2903. CrossRef - Preoperative Serum Thyroglobulin and Its Correlation with the Burden and Extent of Differentiated Thyroid Cancer
Hosu Kim, So Young Park, Jun-Ho Choe, Jee Soo Kim, Soo Yeon Hahn, Sun Wook Kim, Jae Hoon Chung, Jaehoon Jung, Tae Hyuk Kim
Cancers.2020; 12(3): 625. CrossRef - Estimating the Growth Rate of Lung Metastases in Differentiated Thyroid Carcinoma: Response Evaluation Criteria in Solid Tumors or Doubling Time?
Eyun Song, Jonghwa Ahn, Min Ji Jeon, Sang Min Lee, Jeong Hyun Lee, Tae Yong Kim, Jung Hwan Baek, Won Bae Kim, Young Kee Shong, Won Gu Kim
Thyroid.2020; 30(3): 418. CrossRef - Impact of delayed radioiodine therapy in intermediate‐/high‐risk papillary thyroid carcinoma
Mijin Kim, Minkyu Han, Min Ji Jeon, Won Gu Kim, In Joo Kim, Jin‐Sook Ryu, Won Bae Kim, Young Kee Shong, Tae Yong Kim, Bo Hyun Kim
Clinical Endocrinology.2019; 91(3): 449. CrossRef - Tertiary Care Experience of Sorafenib in the Treatment of Progressive Radioiodine-Refractory Differentiated Thyroid Carcinoma: A Korean Multicenter Study
Mijin Kim, Tae Hyuk Kim, Dong Yeob Shin, Dong Jun Lim, Eui Young Kim, Won Bae Kim, Jae Hoon Chung, Young Kee Shong, Bo Hyun Kim, Won Gu Kim
Thyroid.2018; 28(3): 340. CrossRef - A Follow-Up Strategy for Patients with an Excellent Response to Initial Therapy for Differentiated Thyroid Carcinoma: Less Is Better
Min Ji Jeon, Mijin Kim, Suyeon Park, Hye-Seon Oh, Tae Yong Kim, Won Bae Kim, Young Kee Shong, Won Gu Kim
Thyroid.2018; 28(2): 187. CrossRef - Preoperative serum thyroglobulin predicts initial distant metastasis in patients with differentiated thyroid cancer
Hosu Kim, Young Nam Kim, Hye In Kim, So Young Park, Jun-Ho Choe, Jung-Han Kim, Jee Soo Kim, Jae Hoon Chung, Tae Hyuk Kim, Sun Wook Kim
Scientific Reports.2017;[Epub] CrossRef
- Clinical Study
- Usefulness of Measuring Thyroid Stimulating Antibody at the Time of Antithyroid Drug Withdrawal for Predicting Relapse of Graves Disease
-
Hyemi Kwon, Won Gu Kim, Eun Kyung Jang, Mijin Kim, Suyeon Park, Min Ji Jeon, Tae Yong Kim, Jin-Sook Ryu, Young Kee Shong, Won Bae Kim
-
Endocrinol Metab. 2016;31(2):300-310. Published online April 25, 2016
-
DOI: https://doi.org/10.3803/EnM.2016.31.2.300
-
-
4,963
View
-
87
Download
-
20
Web of Science
-
19
Crossref
-
 Abstract Abstract
 PDF PDF PubReader PubReader
- Background
Hyperthyroidism relapse in Graves disease after antithyroid drug (ATD) withdrawal is common; however, measuring the thyrotropin receptor antibody (TRAb) at ATD withdrawal in order to predict outcomes is controversial. This study compared measurement of thyroid stimulatory antibody (TSAb) and thyrotropin-binding inhibitory immunoglobulin (TBII) at ATD withdrawal to predict relapse. MethodsThis retrospective study enrolled patients with Graves disease who were treated with ATDs and whose serum thyroid-stimulating hormone levels were normal after receiving low-dose ATDs. ATD therapy was stopped irrespective of TRAb positivity after an additional 6 months of receiving the minimum dose of ATD therapy. Patients were followed using thyroid function tests and TSAb (TSAb group; n=35) or TBII (TBII group; n=39) every 3 to 6 months for 2 years after ATD withdrawal. ResultsTwenty-eight patients (38%) relapsed for a median follow-up of 21 months, and there were no differences in baseline clinical characteristics between groups. In the TSAb group, relapse was more common in patients with positive TSAb at ATD withdrawal (67%) than patients with negative TSAb (17%; P=0.007). Relapse-free survival was shorter in TSAb-positive patients. In the TBII group, there were no differences in the relapse rate and relapse-free survivals according to TBII positivity. For predicting Graves disease relapse, the sensitivity and specificity of TSAb were 63% and 83%, respectively, whereas those of TBII were 28% and 65%. ConclusionTSAb at ATD withdrawal can predict the relapse of Graves hyperthyroidism, but TBII cannot. Measuring TSAb at ATD withdrawal can assist with clinical decisions making for patients with Graves disease.
-
Citations
Citations to this article as recorded by  - Analysis of Related Factors in Refractory Graves’ Disease
鑫 王
Advances in Clinical Medicine.2023; 13(08): 13439. CrossRef - Interpretation of Thyroid Autoantibodies in Hyperthyroidism
Han-Sang Baek, Dong-Jun Lim
The Korean Journal of Medicine.2023; 98(3): 132. CrossRef - The Early Changes in Thyroid-Stimulating Immunoglobulin Bioassay over Anti-Thyroid Drug Treatment Could Predict Prognosis of Graves’ Disease
Jin Yu, Han-Sang Baek, Chaiho Jeong, Kwanhoon Jo, Jeongmin Lee, Jeonghoon Ha, Min Hee Kim, Jungmin Lee, Dong-Jun Lim
Endocrinology and Metabolism.2023; 38(3): 338. CrossRef - Thyroid-Stimulatory Antibody as a Predictive Factor for Graves’ Disease Relapse
Tiago Da Silva Santos, José Carlos Oliveira, Cláudia Freitas, André Couto de Carvalho
Cureus.2022;[Epub] CrossRef - The Prediction Model Using Thyroid-stimulating Immunoglobulin Bioassay For Relapse of Graves’ Disease
Han-Sang Baek, Jaejun Lee, Chai-Ho Jeong, Jeongmin Lee, Jeonghoon Ha, Kwanhoon Jo, Min-Hee Kim, Jae Hyoung Cho, Moo Il Kang, Dong-Jun Lim
Journal of the Endocrine Society.2022;[Epub] CrossRef - Identification of patients with Graves’ disease who benefit from high-dose radioactive iodine therapy
Shiro Watanabe, Shozo Okamoto, Kazumasa Akikawa, Noriyuki Miyamoto, Miyuki Okamura-Kawasaki, Yuko Uchiyama, Junki Takenaka, Takuya Toyonaga, Kenji Hirata, Kohsuke Kudo
Annals of Nuclear Medicine.2022; 36(11): 923. CrossRef - The relationship between atherosclerotic disease and relapse during ATD treatment
Xinxin Zhu, Yaguang Zhang, Xiaoyu Zhao, Xiaona Zhang, Zixuan Ru, Yanmeizhi Wu, Xu Yang, Boyu Hou, Hong Qiao
Frontiers in Cardiovascular Medicine.2022;[Epub] CrossRef - Antithyroid Drug Treatment in Graves’ Disease
Jae Hoon Chung
Endocrinology and Metabolism.2021; 36(3): 491. CrossRef - The prognostic value of thyroid-stimulating immunoglobulin in the management of Graves’ disease
Yulin Zhou, Mengxi Zhou, Yicheng Qi, Weiqing Wang, Xinxin Chen, Shu Wang
Therapeutic Advances in Endocrinology and Metabolism.2021; 12: 204201882110449. CrossRef - Changes in Thyroid Peroxidase and Thyroglobulin Antibodies Might Be Associated with Graves' Disease Relapse after Antithyroid Drug Therapy
Yun Mi Choi, Mi Kyung Kwak, Sang Mo Hong, Eun-Gyoung Hong
Endocrinology and Metabolism.2019; 34(3): 268. CrossRef - Graves' Disease: Can It Be Cured?
Wilmar M. Wiersinga
Endocrinology and Metabolism.2019; 34(1): 29. CrossRef - Medical Treatment of Graves' Disease
Hyun-Kyung Chung
International Journal of Thyroidology.2019; 12(2): 79. CrossRef - When should antithyroid drug therapy to reduce the relapse rate of hyperthyroidism in Graves’ disease be discontinued?
Suyeon Park, Eyun Song, Hye-Seon Oh, Mijin Kim, Min Ji Jeon, Won Gu Kim, Tae Yong Kim, Young Kee Shong, Doo Man Kim, Won Bae Kim
Endocrine.2019; 65(2): 348. CrossRef - Elevated Serum IL-17 Expression at Cessation Associated with Graves’ Disease Relapse
Jianhui Li, Xiaohua Sun, Danzhen Yao, Jinying Xia
International Journal of Endocrinology.2018; 2018: 1. CrossRef - Active Surveillance for Patients With Papillary Thyroid Microcarcinoma: A Single Center’s Experience in Korea
Hyemi Kwon, Hye-Seon Oh, Mijin Kim, Suyeon Park, Min Ji Jeon, Won Gu Kim, Won Bae Kim, Young Kee Shong, Dong Eun Song, Jung Hwan Baek, Ki-Wook Chung, Tae Yong Kim
The Journal of Clinical Endocrinology & Metabolism.2017; 102(6): 1917. CrossRef - Free Thyroxine, Anti-Thyroid Stimulating Hormone Receptor Antibody Titers, and Absence of Goiter Were Associated with Responsiveness to Methimazole in Patients with New Onset Graves' Disease
Hoon Sung Choi, Won Sang Yoo
Endocrinology and Metabolism.2017; 32(2): 281. CrossRef - The Second Antithyroid Drug Treatment Is Effective in Relapsed Graves' Disease Patients: A Median 11-Year Follow-Up Study
Ye An Kim, Sun Wook Cho, Hoon Sung Choi, Shinje Moon, Jae Hoon Moon, Kyung Won Kim, Do Joon Park, Ka Hee Yi, Young Joo Park, Bo Youn Cho
Thyroid.2017; 27(4): 491. CrossRef - The Recurrence Rate of Graves' Disease among Patients with Subclinical Thyrotoxicosis after Initial Remission with Antithyroid Agents
Myoung Sook Shim, Soo Min Nam, Jin Sae Yoo, Hae Kyung Kim, Sang Jun Lee, Mi Young Lee
International Journal of Thyroidology.2017; 10(2): 77. CrossRef - Articles inEndocrinology and Metabolismin 2016
Won-Young Lee
Endocrinology and Metabolism.2017; 32(1): 62. CrossRef
|